Abstract
Despite the recent advance in C–H bond functionalization chemistry, the C–H bonds in the acridine ring system, which is an important scaffold in medicinal and material science, have met with limited success, due, in part, to the lack of activated C–H bonds adjacent to the ring
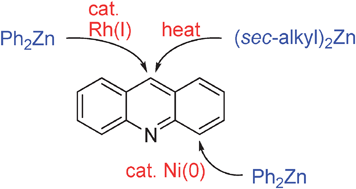
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...