A diastereomerically enriched, dimeric organolithium compound and the stereochemical course of its transformations†
Abstract
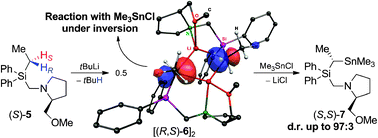
A dimeric α-silylated ethyllithium compound is presented featuring stereogenic metalated carbon centres with defined configurations. It can be generated by diastereoselective deprotonation of the corresponding ethylsilane. Reaction with Me3SnCl proceeds under inversion and the transfer of the stereoinformation is possible with dr's of up to 97 : 3.

 Please wait while we load your content...
Please wait while we load your content...