Selective formation of angular tricyclic compounds by ruthenium-mediated ring-rearrangement metathesis†‡
Abstract
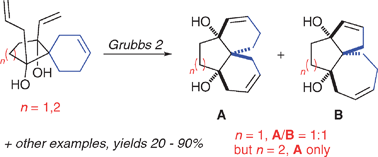
Unsaturated spirocyclic substrates bearing two
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...