Aminoborylation/Suzuki–Miyaura tandem cross coupling of aryl iodides as efficient and selective synthesis of unsymmetrical biaryls†‡
Abstract
Sequential
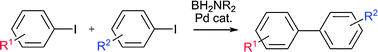
- This article is part of the themed collection: Emerging Investigators 2012
* Corresponding authors
a
Institut des Sciences Moléculaires, UMR CNRS 5255, Université Bordeaux 1, 351 Cours de la Libération, F-33405 Talence Cedex, France
E-mail:
m.pucheault@ism.u-bordeaux1.fr
b Chimie et Photonique Moléculaires, UMR CNRS 6510, Université Rennes 1, Campus de Beaulieu, 263 Avenue du Général Leclerc, F-35042 Rennes Cedex, France
Sequential
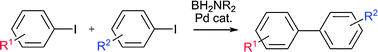
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
L. Marciasini, N. Richy, M. Vaultier and M. Pucheault, Chem. Commun., 2012, 48, 1553 DOI: 10.1039/C1CC14605J
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
