Separation of all-trans- and 13-cis-isomers of acitretin on a reversed-phase column, optimization of stability conditions and their simultaneous quantitation in human plasma by liquid chromatography-tandem mass spectrometry
Abstract
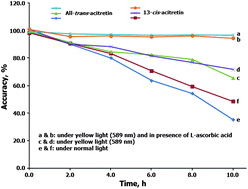
A selective, sensitive and high throughput liquid chromatography-tandem mass spectrometry (LC-ESI-MS/MS) method has been developed and validated for the chromatographic separation and quantitation of all-trans-acitretin and 13-cis-acitretin in human plasma. Sample clean-up involved solid phase extraction of both the isomers and their deuterated analogs (all-trans-acitretin-d3 and 13-cis-acitretin-d3) used as internal standards from 300 μL human plasma. Both the analytes were chromatographically separated with a resolution factor of 2.3 on a Gemini C18 (50 × 2.0 mm, 3 μ) analytical column using 10 mM ammonium acetate and acetonitrile (60 : 40, v/v) as the mobile phase. The selectivity factor (α) of the column for the separation was 1.28, based on the capacity factors of 4.56 and 5.84 for (all-trans) and (13-cis)-isomers respectively. The parent → product ion transitions for both the isomers (m/z 325.2 → 266.0) and IS (m/z 328.0 → 266.0) were monitored on a triple quadrupole mass spectrometer, operating in the multiple reaction monitoring (MRM) and negative ion mode. The method was validated over the concentration range of 0.3–500 ng mL−1 for both the analytes. Stability studies were conducted in plasma to check the extent of photoisomerization under different conditions. Quantitative and consistent recovery (>92%) was obtained in the presence of L-ascorbic acid and yellow light (589 nm) for both the analytes. The matrix effect was assessed by post-column analyte infusion and the precision value (% CV) for a relative matrix effect in six different lots of plasma samples was ≤3.9% at the LLOQ levels for both the analytes. The method was successfully applied to a pivotal bioequivalence study in 48 healthy Indian subjects after oral administration of 25 mg acitretin tablet formulation under fasting conditions. To demonstrate the reproducibility in the measurement of study data, an incurred sample reanalysis was done with 270 subject samples and the % change in concentration from the initial analysis was within ±15%.
 Please wait while we load your content...
Please wait while we load your content...