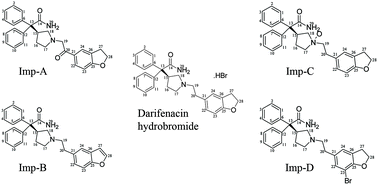
The present study describes the identification and characterization of two process impurities and major stress degradants in darifenacin hydrobromide using high performance liquid chromatography (HPLC) analysis. Forced degradation studies confirmed that the drug substance was stable under acidic, alkaline, aqueous hydrolysis, thermal and photolytic conditions and susceptible only to oxidative degradation. Impurities were identified using liquid chromatography coupled with ion trap mass spectrometry (LC-MS/MSn). Proposed structures were unambiguously confirmed by synthesis followed by characterization using nuclear magnetic resonance spectroscopy (NMR), infrared spectroscopy (IR) and elemental analysis (EA). Based on the spectroscopic, spectrometric and elemental analysis data, the unknown impurities were characterized as 2-{1-[2-(2,3-dihydrobenzofuran-5-yl)-2-oxo-ethyl]-pyrrolidin-3-yl}-2,2-diphenylacetamide (Imp-A), 2-[1-(2-benzofuran-5-yl-ethyl)-pyrrolidin-3-yl]-2,2-diphenylacetamide (Imp-B), 2-{1-[2-(2,3-dihydrobenzofuran-5-yl)-ethyl]-1-oxy-pyrrolidin-3-yl}-2,2-diphenylacetamide (Imp-C) and 2-{1-[2-(7-bromo-2,3-dihydrobenzofuran-5-yl)-ethyl]-pyrrolidin-3-yl}-2,2-diphenylacetamide (Imp-D). Plausible mechanisms for the formation and control of these impurities have also been proposed. The method was validated as per regulatory guidelines to demonstrate specificity, sensitivity, linearity, precision, accuracy and the stability-indicating nature. Regression analysis showed a correlation coefficient value greater than 0.99 for darifenacin hydrobromide and its impurities. The accuracy of the method was established based on the recovery obtained between 86.6 and 106.7% for all impurities.

 Please wait while we load your content...
Please wait while we load your content...