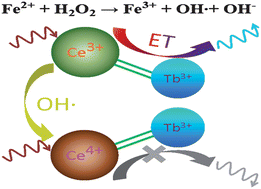
A new method for quenching kinetic discrimination of Fe2+ and Fe3+, and sensitive detection of trace amount of Fe2+ was developed by using synchronous fluorescence scan technique. The principle of this assay is based on the quenching kinetic discrimination of Fe2+ and Fe3+ in CePO4:Tb3+ nanocrytals–H2O2 hybrid system and the Fenton reaction between Fe2+ and H2O2. Stable, water-soluble and well-dispersible CePO4:Tb3+ nanocrystals were synthesized in aqueous solutions, and characterized by transmission electron microscopy (TEM) and electron diffraction spectroscopy (EDS). We found that both Fe2+ and Fe3+ could quench the synchronous fluorescence of CePO4:Tb3+ nanocrytals-H2O2 system, but their quenching kinetics velocities were quite different. In the presence of Fe3+, the synchronous fluorescent intensity was unchanged after only one minute, but in the presence of Fe2+, the synchronous fluorescent intensity decreased slowly until 28 min later. The Fenton reaction between Fe2+ and H2O2 resulted in hydroxyl radicals which effectively quenched the synchronous fluorescence of the CePO4:Tb3+ nanocrystals due to the oxidation of Ce3+ into Ce4+ by hydroxyl radicals. Under optimum conditions, the linear range for Fe2+ is 3 nM–2 μM, and the limit of detection is 2.0 nM. The method was used to analyze water samples.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...