Structural properties of the dipolar hard-sphere fluid at low temperatures and densities
Abstract
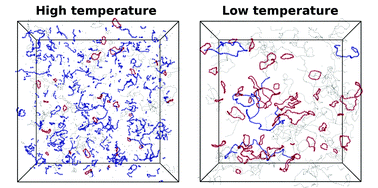
Through extensive state-of-the-art numerical simulations, we study the behavior of the dipolar hard sphere model at low temperatures and low densities, shedding light on a region of the phase diagram where a topological phase transition has long been thought to occur. We show that the system exhibits remarkable and unusual behaviors, like a very low density percolation locus and a stabilization of rings over chain structures. This unexpected abundance of rings comes from a delicate balance between the lower ring energy and the end-to-end chain entropy, and hints at a possible mechanism for the suppression of the gas–liquid phase separation. Our results open the possibility for refined theoretical approaches which, in addition to the previously encompassed chain and branched geometries, must also include the significant contribution arising from ring formation.

 Please wait while we load your content...
Please wait while we load your content...