Au@Pt dendrimer encapsulated nanoparticles as model electrocatalysts for comparison of experiment and theory†
Abstract
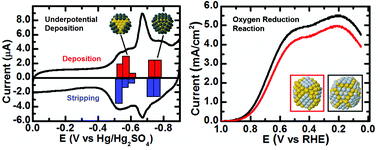
In this paper we report the electrochemical synthesis of core@shell dendrimer-encapsulated nanoparticles (DENs) consisting of cores containing 147 Au atoms (Au147) and Pt shells having ∼54 or ∼102 atoms (Au147@Ptn (n = 54 or 102)). The significance of this work arises from the correlation of the experimentally determined structural and electrocatalytic properties of these particles with density functional theory (DFT) calculations. Specifically, we describe an experimental and theoretical study of Pb underpotential deposition (UPD) on Au147 DENs, the structure of both Au147@Pbn and Au147@Ptn DENs, and the activity of these DENs for the oxygen reduction reaction (ORR). DFT calculations show that Pb binding is stronger on the (100) facets of Au as compared to (111), and the calculated deposition and stripping potentials are consistent with those measured experimentally. Galvanic exchange is used to replace the surface Pb atoms with Pt, and a surface distortion is found for Au147@Ptn particles using molecular dynamics simulations in which the Pt-covered (100) facets shear into (111) diamond structures. DFT calculations of oxygen binding show that the distorted surfaces are the most active for the ORR, and that their activity is similar regardless of the Pt coverage. These calculations are consistent with rotating ring-disk voltammetry measurements.

 Please wait while we load your content...
Please wait while we load your content...