Metallosupramolecular complex targeting an α/β discordant stretch of amyloid β peptide†
Abstract
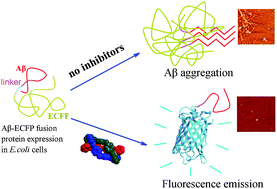
The accumulation of amyloid β-peptide (Aβ) is one of the pathological hallmarks of Alzheimer's disease (AD). Developing Aβ amyloid inhibitors has received much attention. Most reported Aβ inhibitors are small organic molecules or peptides. Here we use a cell-based novel Aβ–enhanced cyan fluorescent protein (ECFP) fluorescent fusion inhibitor screen system, biochemical and biophysical approaches and in vivo studies to identify two zinc-finger-like triple-helical metallo-supramolecular cylinders, [Ni2L3]4+ and [Fe2L3]4+, that can strongly inhibit Alzheimer's disease β-amyloid aggregation. Further studies indicate that the two metallo-supramolecular cylinders are specifically targeting the α/β-discordant stretch and reducing Aβ cytotoxicity. In vivo studies demonstrate that these complexes can ameliorate spatial memory deficits in a transgenic mouse model and decrease the insoluble Aβ level. This is the first demonstration that zinc-finger-like metallo-supramolecular cylinders can be Aβ aggregation inhibitors that specifically target an α/β-discordant stretch. Our work will prompt design and screening of metallo-supramolecular complexes as potential therapeutic agents for AD.

 Please wait while we load your content...
Please wait while we load your content...