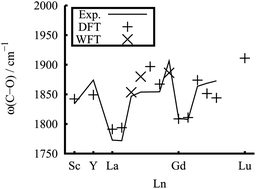
The reactions of Group-3 metal atoms with carbon monoxide in solid argon have been studied using matrix isolation infrared absorption spectroscopy. The lanthanide monocarbonyls LnCO were produced spontaneously on annealing. The observations on LnCO, Ln = Pr, Nd, Sm, Eu, Tb, Dy, Ho, and Er are new. We also theoretically study the structure, bonding, and C–O stretch infrared frequencies. The covalent M–C bonding contains both M ← C σ donation from the carbon lone pair, and M 5d → CO 2π* back donation contributions. In addition to the open 4fn shells, the total spin, as found earlier, may have contributions from an M ‘σ doughnut’ and the M–C π bond, in a σ1π1, σ1π2 high-spin, or σ2π1 low-spin configuration. They form at least a single-bond, and those with the σ1π2 configuration approach a double bond in length. The weakening of the C–O bonding is related to the back donation to the antibonding C–O 2π* orbital.

 Please wait while we load your content...
Please wait while we load your content...