NMR relaxation and structural elucidation of peptides in the presence and absence of trifluoroethanol illuminates the critical molecular nature of integrin αvβ6 ligand specificity†
Abstract
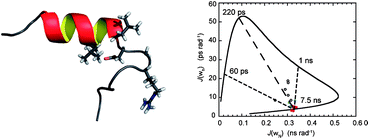
Integrin αvβ6 is an important emerging target for both imaging and therapy of cancer that requires specific ligands based on Arg-Gly-Asp (RGD) peptides. There remains little correlation between integrin-RGD ligand specificity despite studies suggesting an RGD-turn-helix ligand motif is required. Here, we describe the application of 15N NMR relaxation analyses and structure determination of αvβ6 peptide ligands in the presence and absence of trifluoroethanol (TFE) to identify their critical molecular nature that influences specificity, interaction and function. Two linear peptides; one known to demonstrate αvβ6 specificity (FMDV2) and the other based on a natural RGD ligand (LAP2), were compared to two additional peptides based on FMDV2 but cyclised in different positions using a disulphide bond (DBD1 and DBD2). The cyclic adaptation in DBD1 produces a significant alteration in backbone dynamic properties when compared to FMDV2; a potential driver for the loss in αvβ6 specificity by DBD1. The importance of ligand dynamics are highlighted through a comprehensive reduced spectral density and ModelFree analysis of peptide 15N NMR relaxation data and suggest αvβ6 specificity requires the formation of a structurally rigid helix preceded by a RGD motif exhibiting slow internal motion. Additional observations include the effect of TFE/water viscosity on global NMR dynamics and the advantages of using spectral density NMR relaxation data to estimate correlation times and motional time regimes for peptides in solution.

 Please wait while we load your content...
Please wait while we load your content...