Activation of serpentine for CO2 mineralization by flux extraction of soluble magnesium salts using ammonium sulfate†
Abstract
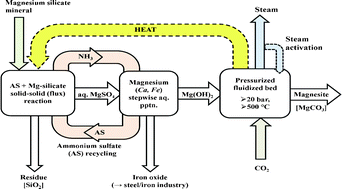
This paper concerns the growing role of cheap and potentially recyclable ammonium salts in CO2 mineralization. The powerful hyphenated technique TG-FTIR, along with XRD and ICP-AES, were used to shed light on the underlying chemistry and measure the efficiency of magnesium ion extraction from a Finnish serpentinite in contact with molten ammonium sulfate above 300 °C. From micro- and gram-scale tests, flux extraction as epsomite [MgSO4·7H2O] proceeds via the intermediacy of Tutton salts, NH4/Mg double sulfates increasingly rich in Mg. Extraction is effected through the agency of acidic derivatives, principally ammonium bisulfate and sulfamic acid, which are created sequentially from ammonium sulfate in situ. However, sulfamic acid volatilizes and/or decomposes at a significant rate by 400 °C. This loss mechanism is primarily responsible for the modest recovery of Mg (50–60%). An improved process would operate below 400 °C where Mg extraction as efremovite [(NH4)2Mg2(SO4)3] is effective. Future experiments evaluating the use of humid air to stabilize the bisulfate and impede the loss of flux are recommended.

 Please wait while we load your content...
Please wait while we load your content...