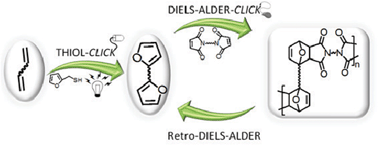
Monomers based on plant oil derivatives bearing furan heterocycles appended through thiol-ene click chemistry were prepared and, subsequently, polymerized via a second type of click reaction, i.e. the Diels–Alder (DA) polycondensation between furan and maleimide complementary moieties. Two basic approaches were considered for these DA polymerizations, namely (i) the use of monomers with two terminal furan rings in conjunction with bismaleimides (AA + BB systems) and (ii) the use of a protected AB monomer incorporating both furan and maleimide end groups. This study clearly showed that both strategies were successful, albeit with different outcomes, in terms of the nature of the ensuing products. The application of the retro-DA reaction to these polymers confirmed their thermoreversible character, i.e. the clean-cut return to their respective starting monomers, opening the way to original macromolecular materials with interesting applications, like mendability and recyclability.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...