Synthesis, photophysical and electrochemical properties of conjugated polymers incorporating 9,9-dialkyl-1,4-fluorenylene units with thiophene, carbazole and triarylamine comonomers†
Abstract
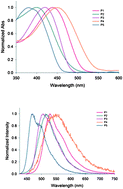
Three new poly(p-phenylenevinylene) derivatives were synthesized via the Horner–Wittig condensation

 Please wait while we load your content...
Please wait while we load your content...