Control of reactivity of constitutional isomers of pentafluorophenyl ethynylbenzoates for the synthesis of functional poly(phenylacetylenes)†
Abstract
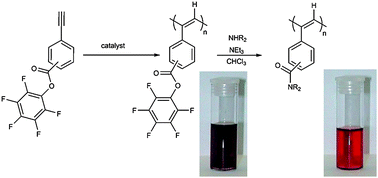
Two series of constitutional isomers of monosubstituted ethynylbenzoates were synthesized. The series of the constitutional isomers were based on methyl ethynylbenzoates (M1–M3) and pentafluorophenyl ester ethynylbenzoates (M4–M6), respectively. Rhodium-based catalysts as well as a tungsten-based catalyst were used to polymerize the monomers M1–M6. While the tungsten catalyst could successfully polymerize all six monomers, the ortho-substituted active ester monomer M4 could not be polymerized with rhodium based catalysts. The polymers were obtained in variable molecular weights ranging from 7000 to 200 000 g mol−1 with molecular weight distributions smaller than 3.6 (mostly Mw/Mn < 1.9). The configuration of the polymeric backbone was characterized by 1H NMR and Raman spectroscopy and it was found that polymers synthesized using the rhodium catalyst possessed a cis–transoid structure, while polymers synthesized with tungsten based catalysts featured a trans–transoid sequence as their major structure. Further, post-polymerization modifications of the reactive polymers with various amines have been investigated and it was found that the polymers showed a different reactivity towards amines depending on the isomer, i.e. the position of the ester moiety at the phenyl ring. Moreover, the UV-vis spectra of the polymers before and after conversion with amines revealed a distinct shift of the UV-vis band maximum of up to 150 nm, accompanied by an apparent colour change indicating a pronounced effect of the side-group onto the conjugated polymer backbone.
- This article is part of the themed collection: New methods of polymer synthesis

 Please wait while we load your content...
Please wait while we load your content...