Porphyrin dyads linked by a rotatable 3,3′-biphenyl scaffold: a new binding motif for small ditopic molecules†‡
Abstract
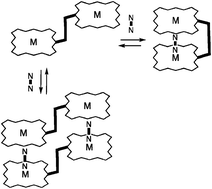
Synthetic access to a set of metallo- and free base bis-porphyrins has been provided by a stepwise approach involving sequential
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...