Metal-free reactions of alkynes via electrophilic iodocarbocyclizations†
Abstract
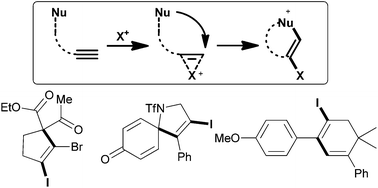
Despite attracting tremendous interest over the last few decades, the field of electrophilic cyclizations is still continuously and rapidly developing. Particularly, metal-free reactions that involve the activation of an alkyne using electrophilic halogen sources are powerful tools in the repertoire of synthetic chemists. This brief overview highlights recent progress in C–C bond-forming halocyclizations allowing for the reaction of alkynes with carbon-nucleophiles. Primarily guided by the type of carbon nucleophile, methods are categorized as the addition of arene, malonate, and olefin nucleophiles.

 Please wait while we load your content...
Please wait while we load your content...