Synthetic approach to flavanones and flavonesvialigand-free palladium(ii)-catalyzed conjugate addition of arylboronic acids to chromones†
Abstract
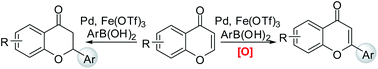
The remarkable catalytic effects of Fe(OTf)3 in the context of the Pd(II)-catalyzed conjugate addition of arylboronic acids to

 Please wait while we load your content...
Please wait while we load your content...