Synthesis of the O-linked hexasaccharide containing β-d-Galf-(1→2)-β-d-Galf in Trypanosoma cruzi mucins†
Abstract
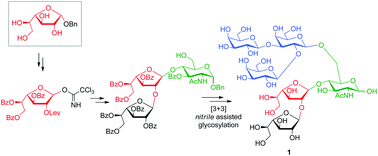
The hexasaccharide β-D-Galp-(1→2)-[β-D-Galp-(1→3)]-β-D-Galp-(1→6)-[β-D-Galf(1→2)-β-D-Galf(1→4)]-D-GlcNAc (1) is the largest carbohydrate structure released as alditol by reductive β-elimination from mucins of some strains of T. cruzi. The terminal β-D-Galp units are sites of sialylation by trans-sialidase which transfers sialic acid from the host to the parasite. Hexasaccharide 1 was synthesized by a [3 + 3]-convergent strategy based on a nitrile assisted glycosylation, using the trichloroacetimidate method. The β-D-Galf-(1→2)-β-D-Galf-D-GlcNAc synthon was sequentially constructed from the reducing end to the non-reducing end employing benzyl α-D-galactofuranoside as starting material for the internal Galf unit. The choice of this novel precursor, obtained in one-reaction step from galactose, allowed the introduction of an orthogonal and participating levulinoyl group at O-2. Thus, the diastereoselective construction of the Galf-β(1→4)-GlcNAc linkage by the trichloroacetimidate method of glycosylation was achieved. The 1H NMR spectrum of alditol 2 was identical to the product released by β-elimination from the parasite mucin.

 Please wait while we load your content...
Please wait while we load your content...