Abstract
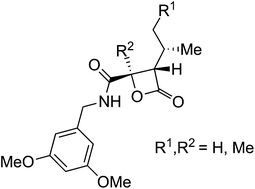
Successful biochemical studies of the natural products belactosin A and C and their acylated congeners have shown a β-lactonecarboxamide moiety to be a possible core structure of powerful proteasome inhibitors. As a part of further investigations, variously decorated simplified β-lactonecarboxamides have been synthesized in order to understand structure–biological activity relations in detail, to find ways of improving their biological activity and stability and to reduce the complexity of their preparation. Biological tests showed that the best compounds possess a high potential against phytopathogenic fungi in the greenhouse.

 Please wait while we load your content...
Please wait while we load your content...