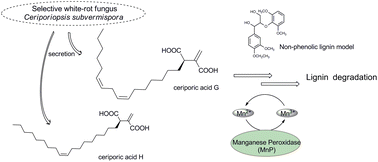
Alkadienyl and alkenyl itaconic acids (ceriporic acids G and H) from the selective white-rot fungus Ceriporiopsis subvermispora: a new class of metabolites initiating ligninolytic lipid peroxidation†
Abstract
New ceriporic acids—alkadienyl and alkenyl itaconic acids having a bis-allyl (

 Please wait while we load your content...
Please wait while we load your content...