Synthesis and applications of masked oxo-sulfinamides in asymmetric synthesis
Abstract
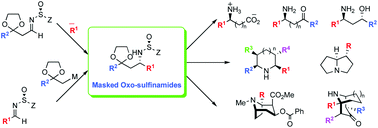
This short perspective reports on the synthesis and applications of a class of chiral amino carbonyl compounds, masked oxo-sulfinamides where the amine is protected with an N-sulfinyl moiety and the carbonyl group is protected as the ketal or 1,3-dithiane. These polyfunctionalized chiral building blocks are prepared by addition of organometallic reagents to masked oxo-sulfinimines (N-sulfinyl imines) or the addition of oxo-organometallic reagents and lithio-1,3-dithianes to sulfinimines. Because unmasking of the amino and carbonyl groups results in cyclic imines, these chiral building blocks are particularly useful for the asymmetric synthesis of functionalized nitrogen heterocycles, including prolines, pipecolic acids, pyrrolidines, homotropinones, tropinones, and tropane alkaloids such as cocaine and C-1 cocaine analogues.

 Please wait while we load your content...
Please wait while we load your content...