Convergent diastereoselective preparation of adjacent quaternary stereocenters in an acyclic system†‡
Abstract
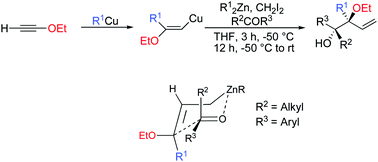
The combined carbometalation–zinc homologation–allylation reaction of the resulting stereodefined 3,3-disubstituted allylmetal species with
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...