Lewis acid-mediated radical cyclization: stereocontrol in cascade radical addition–cyclization–trapping reactions†
Abstract
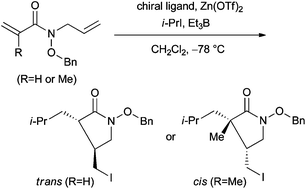
An efficient approach for achieving radical cyclizations by using hydroxamate ester as a coordination tether with Lewis acid was studied. The chiral Lewis acid-mediated cascade radical addition–

 Please wait while we load your content...
Please wait while we load your content...