Enantioselective synthesis of C-linked spiroacetal-triazoles as privileged natural product-like scaffolds†‡
Abstract
The enantioselective synthesis of novel C-linked spiroacetal-
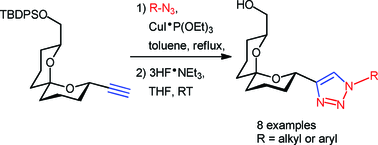
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...