Retracted Article: Organocatalytic stereoselective synthesis of passifloricin A†
Abstract
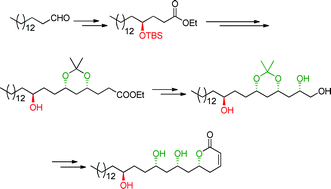
The enantioselective synthesis of passifloricin A has been achieved in high diastereomeric excess. The 1,3-polyol moiety was constructed by iterative proline-catalyzed sequential α-aminoxylation and Horner–Wadsworth–Emmons (HWE) olefination of

 Please wait while we load your content...
Please wait while we load your content...