2, 2′-(phenylazanediyl) diacetic acid modified Fe3O4@PEI for selective removal of cadmium ions from blood†
Abstract
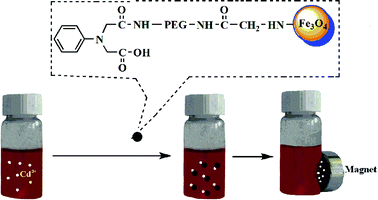
A water-dispersible and supermagnetic nanocomposite (PAD-PEG-Fe3O4@PEI) has been successfully synthesized using polyethylenimine (PEI, Mol MW = 10000) coated supermagnetic Fe3O4–NH2 which was modified with 2, 2′-(phenylazanediyl) diacetic acid (PAD) through the bridge of poly(ethylene glycol) (PEG, Mol MW = 2000). The average particle size of PAD-PEG-Fe3O4@PEI was determined by

 Please wait while we load your content...
Please wait while we load your content...