Enantiomeric pairs reveal that key medicinal chemistry parameters vary more than simple physical property based models can explain†
Abstract
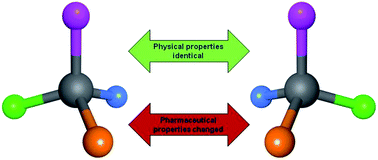
An on-going trend in the medicinal chemistry literature is to link physical properties of a molecule that are achiral (such as lipophilicity) to biological or

 Please wait while we load your content...
Please wait while we load your content...