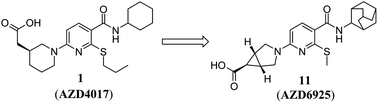
Reduction of acyl glucuronidation in a series of acidic 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors: the discovery of AZD6925†
Abstract
Inhibition of 11β-HSD1 is viewed as a potential target for the treatment of obesity and other elements of the metabolic syndrome. We report here the optimisation of a

 Please wait while we load your content...
Please wait while we load your content...