Synthesis and optical properties of Li3Ba2La3(MoO4)8:Eu3+ powders and ceramics for pcLEDs†
Abstract
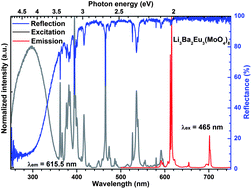
A series of polycrystalline Li3Ba2La3−xEux(MoO4)8 samples were prepared by the conventional solid-state reaction. The phase formation of the samples was investigated by

 Please wait while we load your content...
Please wait while we load your content...