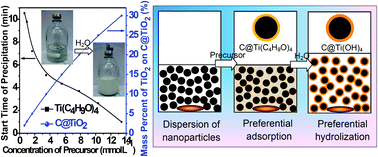
In this study, an effective method of slow hydrolization of metal alkoxide (e.g., Ti(C4H9O)4) in an ethanol–water system was systematically investigated and used to finely control the deposition of titania on carbon colloids. A model of adsorption–hydrolization of precursors during the coating process was rationally built for the first time to interpret the usability of the method and facilitate its further extension. Using this strategy, titania in the form of supported nanocrystals or layers on carbon colloids (TiO2/C, C@TiO2) was successfully tailored. Meanwhile, finely dispersed hollow TiO2 nanoparticles with shells consisting of different crystalline structures were also prepared by varying the calcination conditions after removing the carbon cores. More importantly, the effects of the crystalline and nano/macrostructures of the as-prepared TiO2 samples in photocatalysis and lithium-ion battery applications were analyzed in detail. The preliminary results show that anatase–rutile TiO2 hollow particles demonstrate a higher catalytic activity in the photo-degradation of rhodamine B than anatase TiO2 hollow particles, powders, and P25. However, in the case of Li-ion battery applications, the anatase TiO2 hollow particles exhibited better performance as anode materials with high capacities of around 190 mA h g−1, 140 mA h g−1, and 120 mA h g−1 at current densities of 60 mA g−1, 120 mA g−1, and 300 mA g−1, respectively, accompanied by stable cyclability.

 Please wait while we load your content...
Please wait while we load your content...