Paper-immobilized enzyme as a green microstructured catalyst†
Abstract
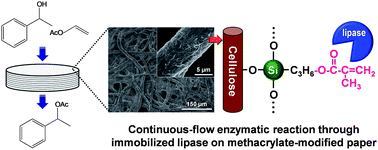
The facile and direct introduction of methacryloxy groups into cellulose paper was carried out using a
* Corresponding authors
a
Department of Biomaterials Sciences, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan
E-mail:
ahkoga@mail.ecc.u-tokyo.ac.jp
Fax: +81-3-5841-5271
Tel: +81-3-5841-5271
b Department of Agro-environmental Sciences, Graduate School of Bioresource and Bioenvironmental Sciences, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan
c Biotron Application Center, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan
The facile and direct introduction of methacryloxy groups into cellulose paper was carried out using a

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
H. Koga, T. Kitaoka and A. Isogai, J. Mater. Chem., 2012, 22, 11591 DOI: 10.1039/C2JM30759F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
