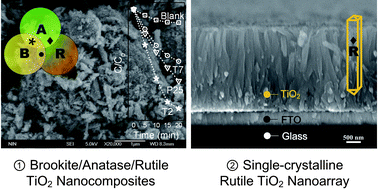
The synthesis of nano-TiO2 materials have attracted intense interest due to their importance in a wide area of applications. In this study, we report a facile method to synthesize mixed-phase TiO2 nanocomposites by using a one-step approach under mild solvothermal conditions. Differently from previous reports, this method not only yields rutile/brookite/anatase TiO2 nanocomposites with high photocatalytic activities, but also can obtain highly oriented single-crystal rutile nanorod arrays selectively deposited on FTO. These products were characterized by XRD, FTIR, FESEM, TEM, HRTEM, and BET. Results indicate that in the brookite/anatase/rutile coexisting nanopowders, the brookite and anatase phases were crystallized into irregular nanoparticles <20 nm in diameter, whereas the rutile phase was crystallized into single-crystalline nanorods ∼20 nm in diameter and 100 to 500 nm in length. The single-crystalline rutile nanorods could form a film with controllable thickness up to ∼7 μm. The sample with 29.9% anatase, 27.9% brookite, 42.2% rutile was shown to have the highest photocatalytic activity, yielding over 90% bleaching of methyl orange solution in 20 min. The degradation rate constant k of this sample was 0.10180 min−1, almost twice as high as that of P25 (k = 0.05397 min−1). DFT calculations were used to confirm the band structures and density of states in brookite, anatase, and rutile phases.

 Please wait while we load your content...
Please wait while we load your content...