One-pot synthesis of hexagonal and triangular nickel–copper alloy nanoplates and their magnetic and catalytic properties†
Abstract
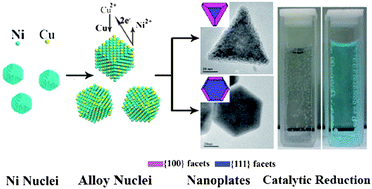
A facile one-pot route has been developed for the synthesis of hexagonal and triangular Ni–Cu alloy nanoplates. The synthesis was conducted using

 Please wait while we load your content...
Please wait while we load your content...