Oxygen transport and isotopic exchange in iron oxide/YSZ thermochemically-active materials via splitting of C(18O)2 at high temperature studied by thermogravimetric analysis and secondary ion mass spectrometry†
Abstract
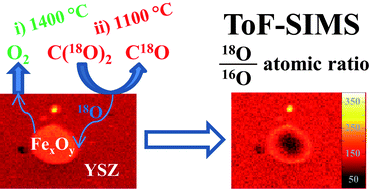
Ferrites are promising materials for enabling solar-thermochemical cycles for the production of synthetic fuels. Such cycles utilize solar-thermal energy for the production of hydrogen from water, or carbon monoxide from carbon dioxide. Recent work studying the thermochemical behaviour of iron oxides co-sintered with yttria-stabilised zirconia (YSZ) using thermogravimetric analysis revealed a striking difference in behaviour of iron that is in solid solution with the YSZ and that which exists as a second iron oxide phase. Materials in which the majority of iron was dissolved in the YSZ exhibited enhanced utilization of iron over those which possessed larger fractions of un-dissolved, bulk iron oxides. To illuminate this phenomena further, several samples of thermally-reduced iron oxide/8YSZ were re-oxidised using isotopically labelled C(18O)2. Post mortem characterization by Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS), with the application of multivariate analysis tools, enables the differentiation between 18O and 16O signals emanating from iron oxide particles. The distribution of 18O is uniform throughout the iron-doped 8YSZ, but concentrated at the surface of iron oxide particles embedded in this matrix. After identical thermal reduction and re-oxidation treatments, the gradient of 18O/16O across the iron oxide particles is found to depend on the size of the iron oxide particles, as well as the method of synthesis of the iron oxide/YSZ material. Comparative thermogravimetric analyses of the 18O-labelled materials and analogous un-labelled materials revealed that exposure to CO2 at 1100 °C results in rapid oxygen isotopic exchange.

 Please wait while we load your content...
Please wait while we load your content...