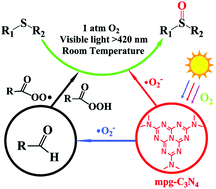
Mesoporous graphitic carbon nitride (mpg-C3N4) has been developed as a non-metal, heterogeneous photocatalyst for the selective oxidation of sulfides to sulfoxides with O2 at room temperature. Especially, the combination of mpg-C3N4 and aldehydes was a highly active system under visible-light irradiation. For example, mpg-C3N4/isobutyraldehyde catalytic oxidation of methyl phenyl sulfide afforded 97% conversion with 98% selectivity for the methyl phenyl sulfoxide in 4 h. Moreover, the mpg-C3N4 can be easily recovered by filtration and then reused at least four times without losing activity. By exploring the electron spin resonance and some comparative experiments, a catalytic mechanism of this oxidation was provided. Finally, the system also works well in the oxidation of a number of sulfides, including sulfides bearing various groups, and phenyl disulfide. The use of a metal-free heterogeneous catalyst and visible light energy, along with the mild reaction conditions makes this oxidation reaction an environmentally benign and energy-saving chemical process.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...