Enzymatic allylic oxidations with a lyophilisate of the edible fungus Pleurotus sapidus†
Abstract
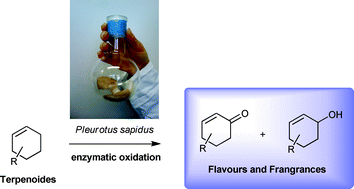
Allylic oxidations belong to the most attractive synthetic transformations because they convert readily available and cheap starting materials into value-added products. In this study, we describe oxidative conversions of

 Please wait while we load your content...
Please wait while we load your content...