Homogeneous catalytic hydrogenation of long-chain esters by an osmium pincer complex and its potential application in the direct conversion of triglycerides into fatty alcohols†
Abstract
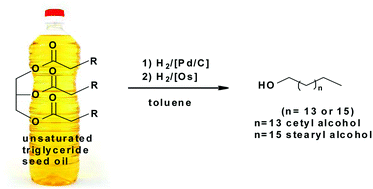
The osmium hydride complexes OsH2(CO)[NH(CH2PiPr2)2] (1) and OsHCl(CO)[NH(CH2PiPr2)2] (2) were evaluated in the catalytic hydrogenation of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, but are subsequently ineffective in the reduction of the ester moiety. However complex 1 is capable of hydrogenating fully saturated
C bonds, but are subsequently ineffective in the reduction of the ester moiety. However complex 1 is capable of hydrogenating fully saturated

 Please wait while we load your content...
Please wait while we load your content...