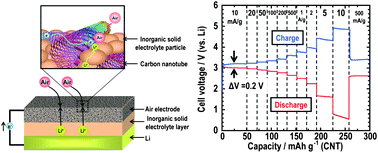
The all-solid-state lithium–air cells using lithium anode, the Li1+xAlyGe2−y(PO4)3 inorganic solid electrolyte and the air electrode composed of carbon nanotubes and inorganic solid electrolyte were constructed. The electrochemical performance and reaction mechanism of the cells were investigated in the air atmosphere. The all-solid-state lithium–air cells were successful in discharging and charging. The first discharge and charge capacities were about 1700 mA h g−1 and 900 mA h g−1, respectively, at a current density of 500 mA g−1 in the voltage range of 2.0–4.2 V (vs. Li/Li+). The observed electrochemical redox potential of around 3.1 V (vs. Li) indicated that the electrochemical reaction in the cells is the formation and decomposition of Li2O2. Additionally, the total polarization of 0.2 V, between charge and discharge curves under a low current density, proved that the use of stable solid electrolytes can improve the large polarization observed in the cell using organic liquid electrolytes. However, the presence of both electrochemical and chemical side reactions, in which gases such as water and carbon dioxide in the ambient air were involved, was also suggested and discussed according to experimental results.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...