Proton-coupled electron transfer kinetics for the hydrogen evolution reaction of hangman porphyrins†‡
Abstract
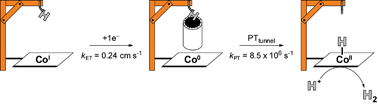
Cobalt hangman
- This article is part of the themed collection: 2023 Journal of Materials Chemistry Lectureship runners-up: Kwabena Bediako and Laure Biniek

 Please wait while we load your content...
Please wait while we load your content...