Synthesis of iridium complexes bearing {o-(Ph2P)C6H4}3E type (E = Si, Ge, and Sn) ligand and evaluation of electron donating ability of group 14 elements E†
Abstract
Trigonal bipyramidal (TBP) iridium(I) complexes {o-(Ph2P)C6H4}3EIr(CO) (E = Si: 1-Ir, Ge: 2-Ir, Sn: 3-Ir) comprising group 14 element E were synthesized and converted into the corresponding cationic iridium(III) complexes [{o-(Ph2P)C6H4}3EIr(H)(CO)][BF4] (E = Si: 4, Ge: 5, Sn: 6) bearing octahedral geometry by protonation using (Et2OH)(BF4). The origin of trans-labilizing abilities of E was investigated through structural analysis, IR and NMR spectroscopic analysis, and density functional theory calculations. Further, the electron-donating abilities of E were investigated through proton transfer reactions.
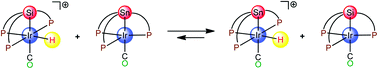

 Please wait while we load your content...
Please wait while we load your content...