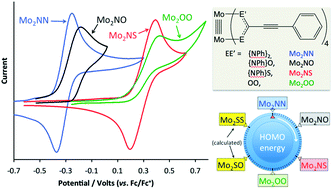
A series of quadruply bonded dimolybdenum compounds of form Mo2(EE′CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)4 (EE′ = {NPh}2, Mo22NN; {NPh}O, Mo22NO;{NPh}S, Mo22NS; OO, Mo22OO) have been synthesised by ligand exchange reactions of Mo2(O2CCH3)4 with the acid or alkali metal salt of {PhC
CPh)4 (EE′ = {NPh}2, Mo22NN; {NPh}O, Mo22NO;{NPh}S, Mo22NS; OO, Mo22OO) have been synthesised by ligand exchange reactions of Mo2(O2CCH3)4 with the acid or alkali metal salt of {PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCEE′}−. The compounds Mo22NO, Mo22NS and Mo22OO were structurally characterised by single crystal X-ray crystallography. The structures show that Mo22NO adopts a cis-2,2 arrangement of the ligands about the Mo24+ core, whereas Mo22NS adopts the trans-2,2 arrangement. The influence of heteroatom substitution on the electronic structure of the compounds was investigated using cyclic voltammetry and UV-Vis spectroscopy. Simple N for O for S substitution in the bridging ligands significantly alters the electronic structure, lowering the energy of the Mo2-δ HOMO and reducing the Mo24+/5+ oxidation potential by up to 0.9 V. A different trend is found in the optoelectronic properties, with the energy of the Mo2-δ-to-ligand-π* transition following the order Mo22OO > Mo22NO > Mo22NN > Mo22NS. Electronic structure calculations employing density functional theory were used to rationalise these observations.
CCEE′}−. The compounds Mo22NO, Mo22NS and Mo22OO were structurally characterised by single crystal X-ray crystallography. The structures show that Mo22NO adopts a cis-2,2 arrangement of the ligands about the Mo24+ core, whereas Mo22NS adopts the trans-2,2 arrangement. The influence of heteroatom substitution on the electronic structure of the compounds was investigated using cyclic voltammetry and UV-Vis spectroscopy. Simple N for O for S substitution in the bridging ligands significantly alters the electronic structure, lowering the energy of the Mo2-δ HOMO and reducing the Mo24+/5+ oxidation potential by up to 0.9 V. A different trend is found in the optoelectronic properties, with the energy of the Mo2-δ-to-ligand-π* transition following the order Mo22OO > Mo22NO > Mo22NN > Mo22NS. Electronic structure calculations employing density functional theory were used to rationalise these observations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)4 (EE′ = {NPh}2, Mo22NN; {NPh}O, Mo22NO;{NPh}S, Mo22NS; OO, Mo22OO) have been synthesised by
CPh)4 (EE′ = {NPh}2, Mo22NN; {NPh}O, Mo22NO;{NPh}S, Mo22NS; OO, Mo22OO) have been synthesised by ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCEE′}−. The compounds Mo22NO, Mo22NS and Mo22OO were structurally characterised by
CCEE′}−. The compounds Mo22NO, Mo22NS and Mo22OO were structurally characterised by 

 Please wait while we load your content...
Please wait while we load your content...