Advanced buckyball joints: synthesis, complex formation and computational simulations of centrohexaindane-extended tribenzotriquinacene receptors for C60 fullerene†
Abstract
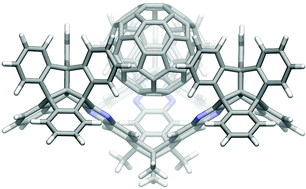
The synthesis of a structurally optimized tribenzotriquinacene receptor 9 is described, which is extended by centrohexaindane moieties to give rise to a half-round concave ball bearing, with optimum shape complementarity towards C60

 Please wait while we load your content...
Please wait while we load your content...