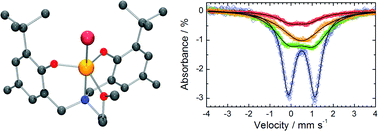
Eight new iron(III) amine–bis(phenolate) complexes are reported. The reaction of anhydrous FeX3 salts (where X = Cl or Br) with the diprotonated tripodal tetradentate ligands 2-tetrahydrofurfurylamino-N,N-bis(2-methylene-4,6-di-tert-butylphenol), H2L1, 2-tetrahydrofurfurylamino-N,N-bis(2-methylene-4-methyl-6-tert-butylphenol), H2L2, and 2-methoxyethylamino-N,N-bis(2-methylene-4,6-di-tert-butylphenol), H2L3, 2-methoxyethylamino-N,N-bis(2-methylene-4-methyl-6-tert-butylphenol), H2L4 produces the trigonal bipyramidal iron(III) complexes, L1FeCl (1a), L1FeBr (1b), L2FeCl (2a), L2FeBr (2b), L3FeCl (3a), L3FeBr (3b), L4FeCl (4a), and L4FeBr (4b). All complexes have been characterized using electronic absorption spectroscopy, cyclic voltammetry and room temperature magnetic measurements. Variable temperature magnetic data were acquired for complexes 2b, 3a and 4b. Variable temperature Mössbauer spectra were obtained for 2b, 3a and 4b. Single crystal X-ray molecular structures have been determined for proligand H2L4 and complexes 1b, 2b, and 4b.

 Please wait while we load your content...
Please wait while we load your content...