Synthesis of quinolinesvia Friedländer reaction catalyzed by CuBTC metal–organic-framework
Abstract
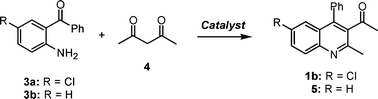
Friedländer condensation between 2-aminoaryl ketones and different
- This article is part of the themed collections: Coordination chemistry in the solid state and In memory of Petr Nachtigall

 Please wait while we load your content...
Please wait while we load your content...