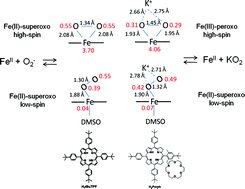
The solution behavior of iron(III) and iron(II) complexes of 54,104,154,204-tetra-tert-butyl-5,10,15,20-tetraphenylporphyrin (H2tBuTPP) and the reaction with superoxide (KO2) in DMSO have been studied in detail. Applying temperature and pressure dependent NMR studies, the thermodynamics of the low-spin/high-spin equilibrium between bis- and mono-DMSO FeII forms have been quantified (KDMSO = 0.082 ± 0.002 at 298.2 K, ΔH° = +36 ± 1 kJ mol−1, ΔS° = +101 ± 4 J K−1 mol−1, ΔV° = +16 ± 2 cm3 mol−1). This is a key activation step for substitution and inner-sphere electron transfer. The superoxide binding constant to the iron(II) form of the studied porphyrin complex was found to be (9 ± 0.5) × 103 M−1, and does not change significantly in the presence of the externally added crown ether in DMSO (11 ± 4) × 103 M−1. The rate constants for the superoxide binding (kon = (1.30 ± 0.01) × 105 M−1 s−1) and release (koff = 11.6 ± 0.7 s−1) are not affected by the presence of the external crown ether in solution. The resulting iron(II)-superoxide adduct has been characterized (mass spectrometry, EPR, high-pressure UV/Vis spectroscopy) and upon controlled addition of a proton source it regenerates the starting iron(II) complex. Based on DFT calculations, the reaction product without neighboring positive charge has iron(II)-superoxo character in both high-spin side-on and low-spin end-on forms. The results are compared to those obtained for the analogous complex with covalently attached crown ether, and more general conclusions regarding the spin-state equilibrium of iron(II) porphyrins, their reaction with superoxide and the electronic structure of the product species are drawn.

 Please wait while we load your content...
Please wait while we load your content...