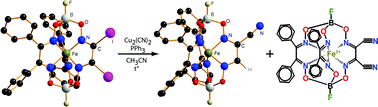
Monoribbed-substituted mono- and dicyano-functionalized iron(II) macrobicycles were obtained for the first time by the reaction of iron(II) diiodoclathrochelate precursor with copper(I) cyanide–triphenylphosphine complex under mild conditions. The target dinitrile clathrochelate is a minor product of this reaction, whereas the major product contains only one cyano group. The clathrochelates obtained were characterized using elemental analysis, 1H and 13C{1H} NMR, IR and UV-vis spectroscopy, MALDI-TOF spectrometry and X-ray diffraction crystallography. The geometry of their FeN6-coordination polyhedra is intermediate between a trigonal prism (TP) and a trigonal antiprism (TAP); the distortion angles, φ, are 22.6–24.7°. In the molecule of the precursor, the Fe–N distances are close, whereas in the mononitrile macrobicycles those for their functionalized chelate fragments are substantially smaller than the corresponding distances in the α-benzyldioximate moieties. The heights, h, of the TP–TAP coordination polyhedra and the average bite angles, α, (2.33 Å and 39°, respectively) are the same for the X-rayed clathrochelates. The UV-vis spectra indicate a dramatic redistribution of the electron density in the π-conjugated clathrochelate framework caused by functionalization with inherent nitrile substituents. The proposed mechanism of the dehalogenation–reduction reaction of iron(II) diiodoclathrochelate resulting in substitution of their iodine atoms by a cyano group and hydrogen atom includes the anion-radical hydrodehalogenation of this precursor with acetonitrile as a source of hydrogen atom. Then, the monomethinemonoiodine macrobicyclic product underwent a substitution with a cyano group only. The copper(I) cyanide–triphenylphosphine–acetonitrile system is proposed as a tool for the synthesis of nitrile derivatives of electron-withdrawing heterocycles starting from their halogen-containing precursors.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...