Reductive dehalogenation of β-haloacrylic ester derivatives mediated by ene-reductases†
Abstract
The enzymatic bioreduction of β-halo-α,β-unsaturated ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C reduction—β-elimination to afford saturated
C reduction—β-elimination to afford saturated
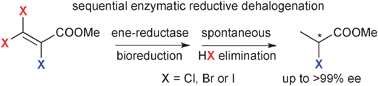
- This article is part of the themed collection: Biocatalysis

 Please wait while we load your content...
Please wait while we load your content...