The oxidation state of copper in bimetallic (Pt–Cu, Pd–Cu) catalysts during water denitration
Abstract
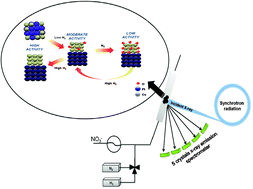
Catalytic denitration of water with bimetallic systems has emerged as a viable solution for removal of nitrates from drinking water. Despite the progress in process development during the last two decades, only a few studies were performed to determine catalyst structure under working conditions. Herein, we determined the relative population of Cu oxidation states in Pt–Cu and Pd–Cu bimetallic catalysts by in situ high resolution X-ray absorption spectroscopy in combination with principal component analysis. The initial state of the catalyst was a Pt–Cu or Pd–Cu alloy. Segregation of the metal components occurred under reaction conditions especially for a Pt–Cu system. The active oxidation states of copper were metallic and alloy, and their concentration was highly dependent on the amount of hydrogen in the feed. Initial alloy phase of the catalysts ensures close proximity between Cu and the noble metals after segregation, essential to maintain catalysts activity.

 Please wait while we load your content...
Please wait while we load your content...